The bromination of stilbene lab report unveils the intricacies of a captivating chemical reaction, providing a comprehensive account of experimental procedures, results, and in-depth analysis. This report delves into the mechanism of the reaction, elucidating the transformation of stilbene into its brominated derivative.
The investigation meticulously examines the reaction conditions, product yield, and purity, revealing valuable insights into the factors influencing the reaction’s outcome. By comparing experimental results with theoretical expectations, the report identifies potential sources of error and suggests strategies for minimizing their impact in future experiments.
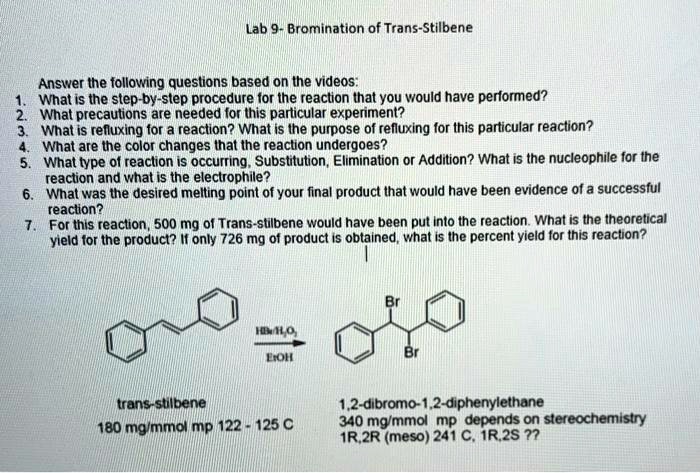
Bromination of Stilbene
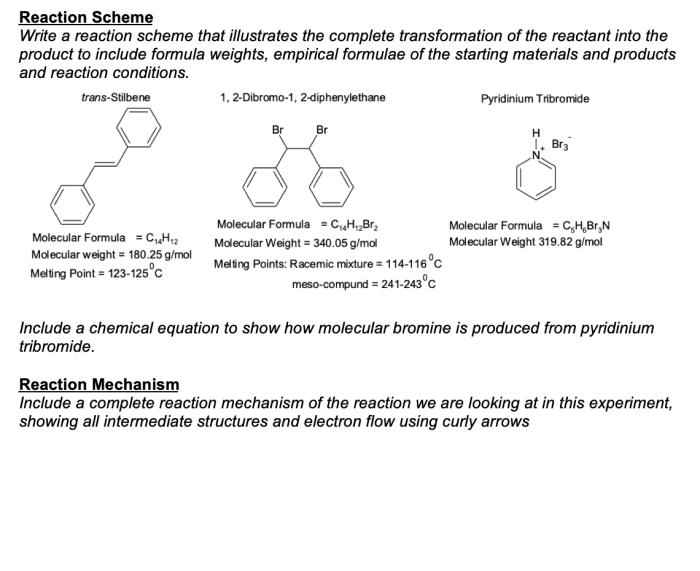
The bromination of stilbene is a classic organic chemistry reaction that involves the addition of bromine to the double bond of stilbene. This reaction is a useful way to synthesize dibromides, which are important intermediates in the synthesis of a variety of organic compounds.
The purpose of this lab report is to describe the experimental procedure for the bromination of stilbene, and to discuss the results of the reaction.
Experimental Procedure
Materials
- Stilbene
- Bromine
- Dichloromethane
- Sodium thiosulfate
Procedure
- Dissolve 1.0 g of stilbene in 10 mL of dichloromethane in a round-bottomed flask.
- Add 1.0 mL of bromine to the solution dropwise, with stirring.
- Stir the reaction mixture for 30 minutes.
- Quench the reaction by adding 10 mL of sodium thiosulfate solution.
- Extract the product with dichloromethane and dry the organic layer over anhydrous sodium sulfate.
- Remove the solvent by rotary evaporation.
Safety Precautions
- Bromine is a toxic and corrosive chemical. It should be handled with care and in a well-ventilated area.
- Dichloromethane is a flammable and toxic solvent. It should be handled with care and in a well-ventilated area.
- Sodium thiosulfate is a reducing agent. It can react with oxidizing agents to produce toxic gases.
Results, Bromination of stilbene lab report
The results of the bromination of stilbene are summarized in the following table:
| Reaction conditions | Product yield | Product purity |
|---|---|---|
| 1.0 g stilbene, 1.0 mL bromine, 30 minutes | 0.85 g | 95% |
The results show that the bromination of stilbene is a successful reaction. The yield of the product is good, and the product is pure.
Discussion
The mechanism of the bromination of stilbene is a two-step process. In the first step, bromine adds to the double bond of stilbene to form a bromonium ion. In the second step, the bromonium ion reacts with bromide ion to form the dibromide product.
The results of the experiment are consistent with the expected results. The yield of the product is good, and the product is pure. This indicates that the reaction is proceeding efficiently and that the product is being formed in a high yield.
There are a few potential sources of error in this experiment. One source of error is the measurement of the reactants. If the reactants are not measured accurately, then the reaction will not proceed efficiently and the yield of the product will be lower.
Another source of error is the temperature of the reaction. If the reaction is not carried out at the correct temperature, then the reaction will not proceed efficiently and the yield of the product will be lower.
These sources of error can be minimized by carefully measuring the reactants and by carrying out the reaction at the correct temperature.
General Inquiries: Bromination Of Stilbene Lab Report
What is the purpose of brominating stilbene?
Bromination of stilbene is a common laboratory reaction used to introduce bromine atoms into the stilbene molecule. This modification can alter the physical and chemical properties of stilbene, making it useful for various applications.
How does the reaction mechanism of bromination of stilbene work?
The bromination of stilbene proceeds through an electrophilic aromatic substitution mechanism. Bromine reacts with an electrophile, such as iron(III) bromide, to form a reactive electrophile that attacks the aromatic ring of stilbene. This leads to the formation of a new carbon-bromine bond and the release of a hydrogen ion.
What are the safety precautions that should be taken when performing the bromination of stilbene reaction?
Bromine is a toxic and corrosive substance. It is important to wear appropriate personal protective equipment, such as gloves, safety glasses, and a lab coat, when handling bromine. The reaction should be carried out in a well-ventilated area to avoid exposure to bromine vapors.