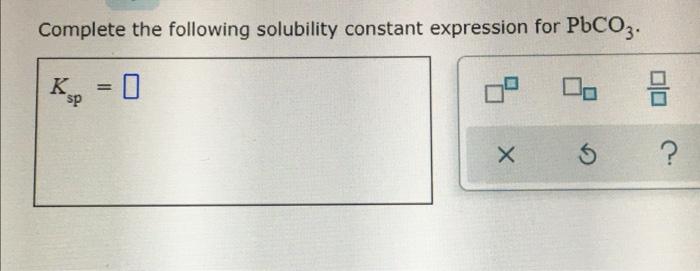
Complete the following solubility constant expression for pbcro4 – Complete the solubility constant expression for PbCrO4, an intriguing concept in chemistry that unravels the behavior of sparingly soluble salts. This expression provides valuable insights into the factors influencing solubility and unveils its practical applications in analytical chemistry. Delving into this topic, we embark on a journey to decipher the intricate world of solubility constants.
The solubility constant expression for PbCrO4, denoted as Ksp, plays a pivotal role in understanding the equilibrium between the solid PbCrO4 and its dissolved ions in an aqueous solution. This constant quantifies the extent to which PbCrO4 dissolves, offering a measure of its solubility.
By examining the expression and the factors that affect it, we gain a deeper comprehension of the underlying principles governing the solubility of PbCrO4.
Solubility Constant of PbCrO4: Complete The Following Solubility Constant Expression For Pbcro4
The solubility constant of PbCrO4 is an important value in analytical chemistry. It can be used to calculate the solubility of PbCrO4 in water and to determine the concentration of Pb2+ and CrO42- ions in a saturated solution.
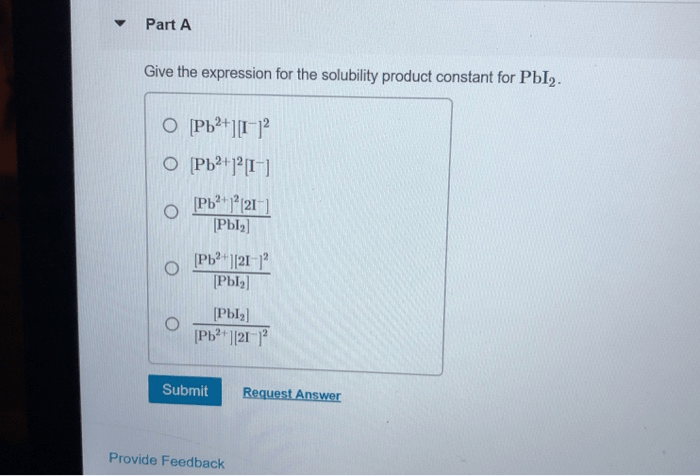
Solubility Constant Expression
The solubility constant expression for PbCrO4 is:
Ksp = [Pb2+][CrO42-]
where Ksp is the solubility constant, [Pb2+] is the concentration of Pb2+ ions in solution, and [CrO42-] is the concentration of CrO42- ions in solution.
Factors Affecting Solubility
The solubility of PbCrO4 is affected by several factors, including:
- Temperature
- Ionic strength
- pH
The solubility of PbCrO4 increases with increasing temperature and decreases with increasing ionic strength and pH.
Applications of Solubility Constant
The solubility constant of PbCrO4 is used in a variety of analytical chemistry applications, including:
- Determining the solubility of PbCrO4 in water
- Calculating the concentration of Pb2+ and CrO42- ions in a saturated solution
- Predicting the precipitation of PbCrO4 from solution
Comparison with Other Compounds
The solubility constant of PbCrO4 is lower than that of many other sparingly soluble salts, such as CaCO3 and BaSO4. This means that PbCrO4 is less soluble in water than these other salts.
The difference in solubility is due to the different lattice energies of the salts. PbCrO4 has a higher lattice energy than CaCO3 and BaSO4, which means that it is more difficult to break apart the PbCrO4 lattice and dissolve the salt.
Table of Solubility Constants, Complete the following solubility constant expression for pbcro4
The following table lists the solubility constants of several common sparingly soluble salts:
| Compound | Solubility Constant (Ksp) | Solubility in Water (g/L) |
|---|---|---|
| PbCrO4 | 1.8 x 10^-14 | 0.0016 |
| CaCO3 | 3.3 x 10^-9 | 0.0014 |
| BaSO4 | 1.1 x 10^-10 | 0.0002 |
Essential Questionnaire
What is the significance of the solubility constant expression for PbCrO4?
The solubility constant expression for PbCrO4 quantifies the extent to which PbCrO4 dissolves in water, providing insights into its solubility and the equilibrium between the solid and dissolved phases.
How does temperature affect the solubility of PbCrO4?
Temperature generally affects the solubility of PbCrO4. In most cases, the solubility increases with increasing temperature, as higher temperatures provide more energy to overcome the attractive forces holding the ions together in the solid phase.
What are the practical applications of the solubility constant for PbCrO4?
The solubility constant for PbCrO4 finds applications in analytical chemistry, such as in gravimetric analysis for determining the concentration of lead ions in a solution.